事業内容
1
医薬品文書
当社では、医学・薬学の専門知識と医薬品開発経験を有するグローバルな専門家ネットワークを活用して、非臨床試験から承認申請、そして市販後調査に至る一連の資料の翻訳とメディカルライティングを行っています。
2
ザイリック
ZYLiQは、米国Symbiance社が開発したウェブベースのアプリケーションです。ZYLiQは、人工知能(AI)を利用して、治験総括報告書(CSR)や科学的文書の作成を自動化します。当社は、ZYLiQの日本での販売代理店として、ZYLiQのプレゼンテーション、受注、納品、導入支援、ユーザーサポート業務を日本語で行っております。
3
国際共通化資料
(CTD)
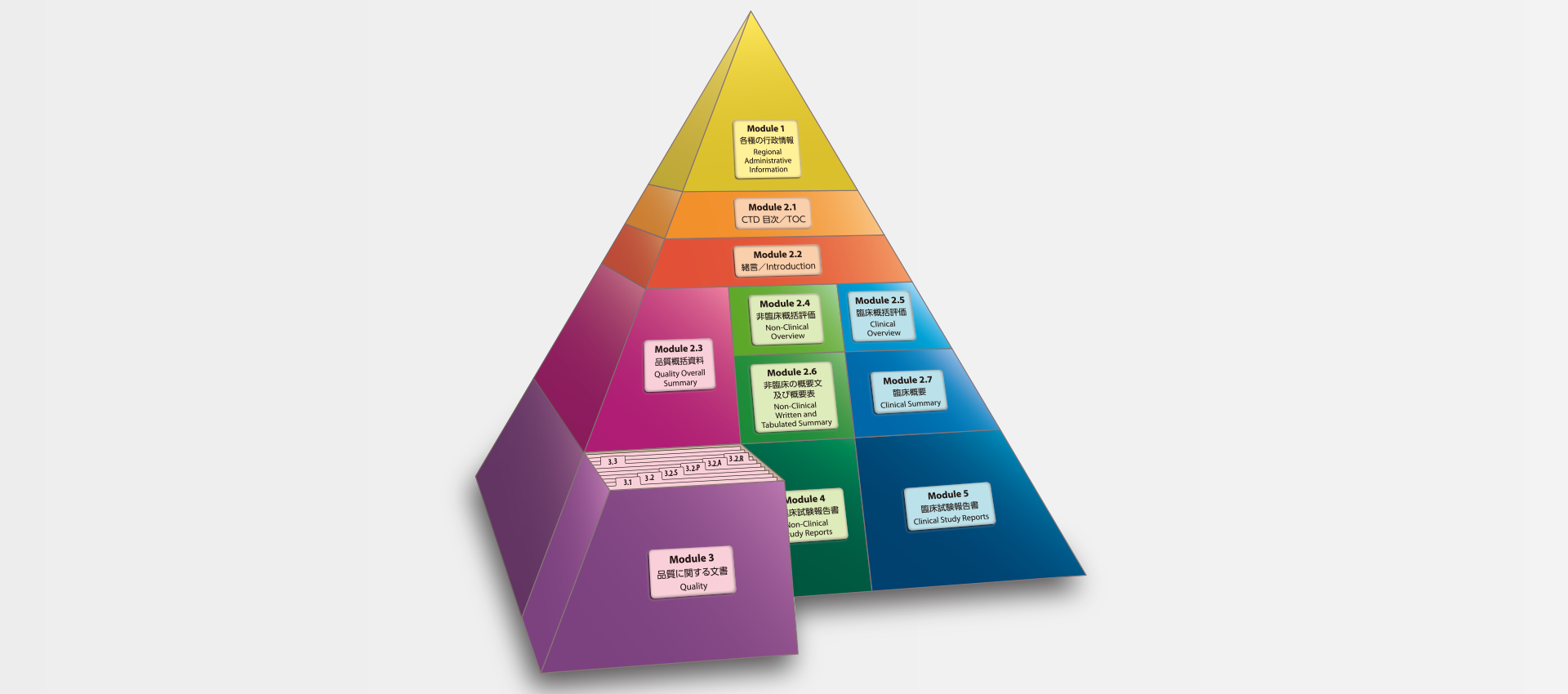
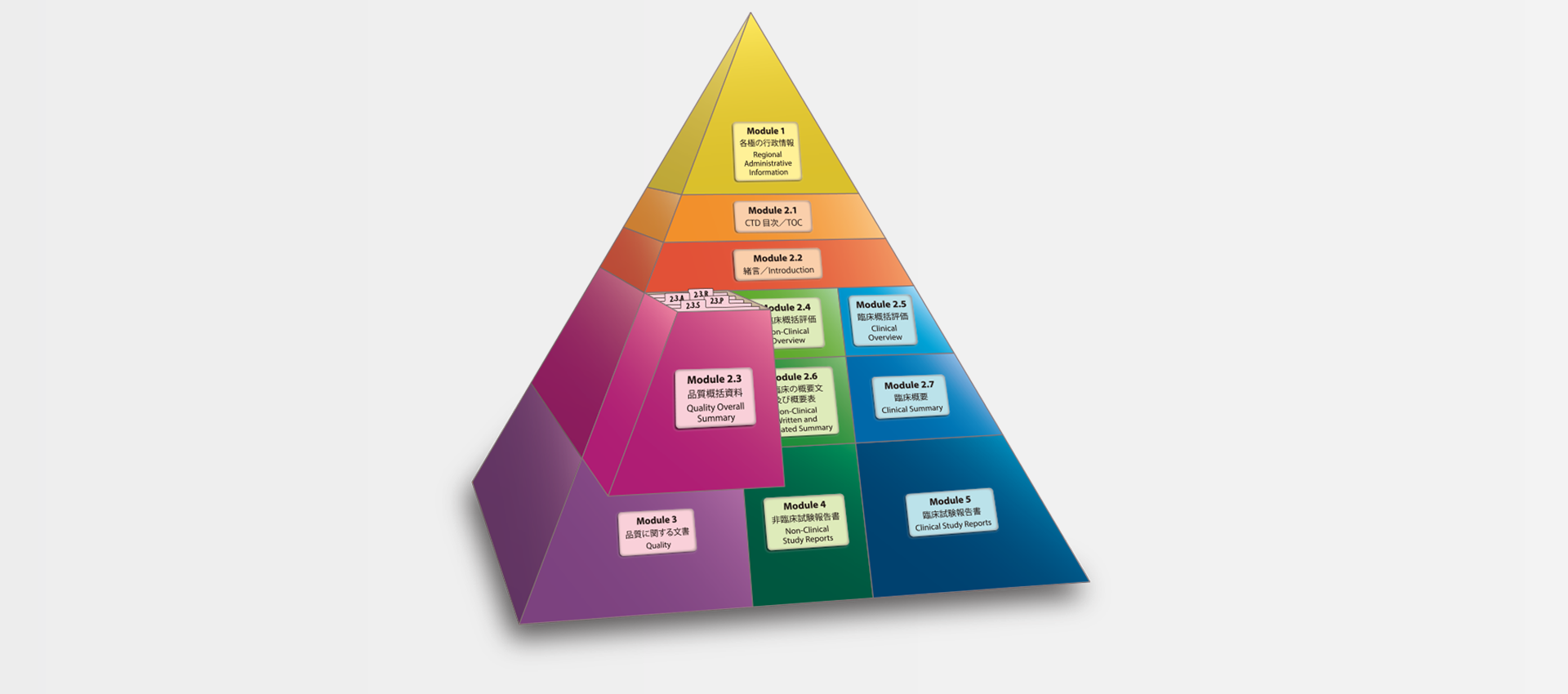
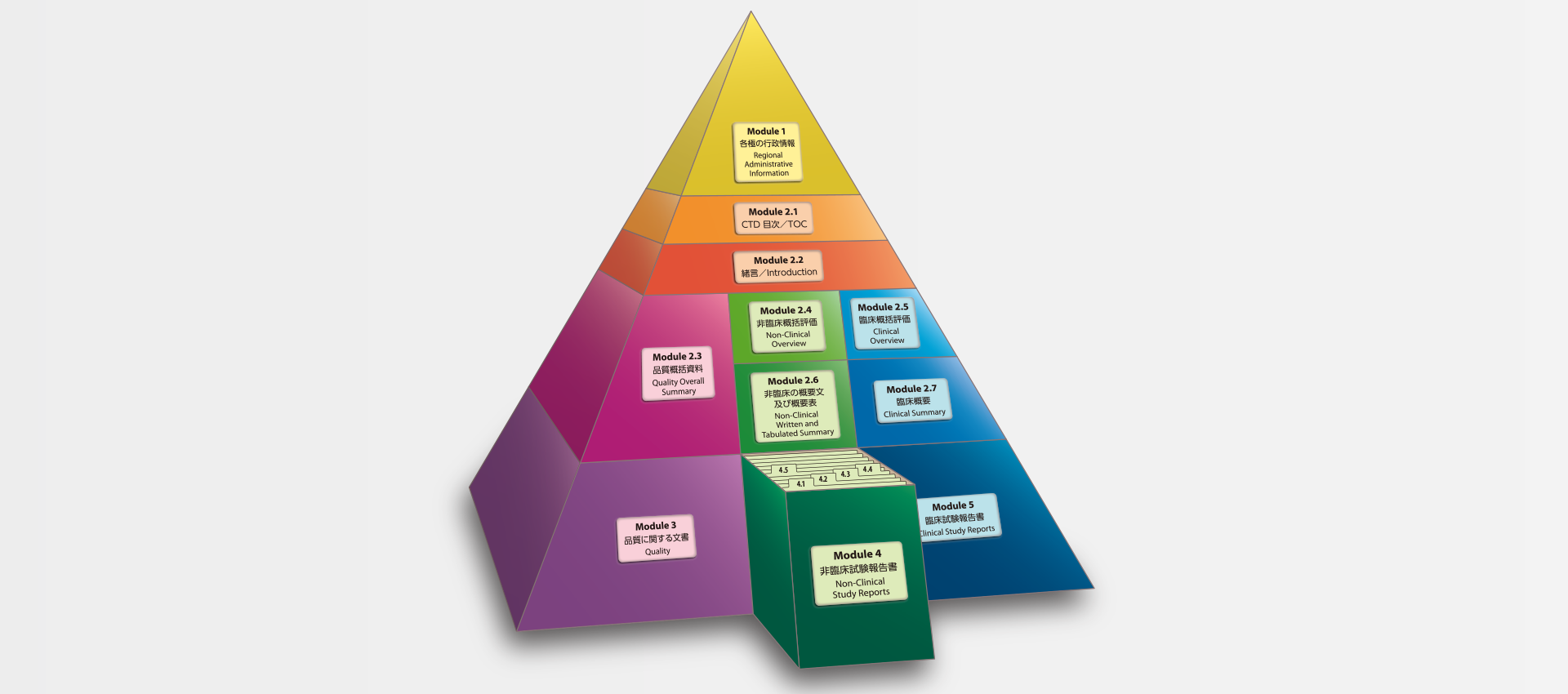
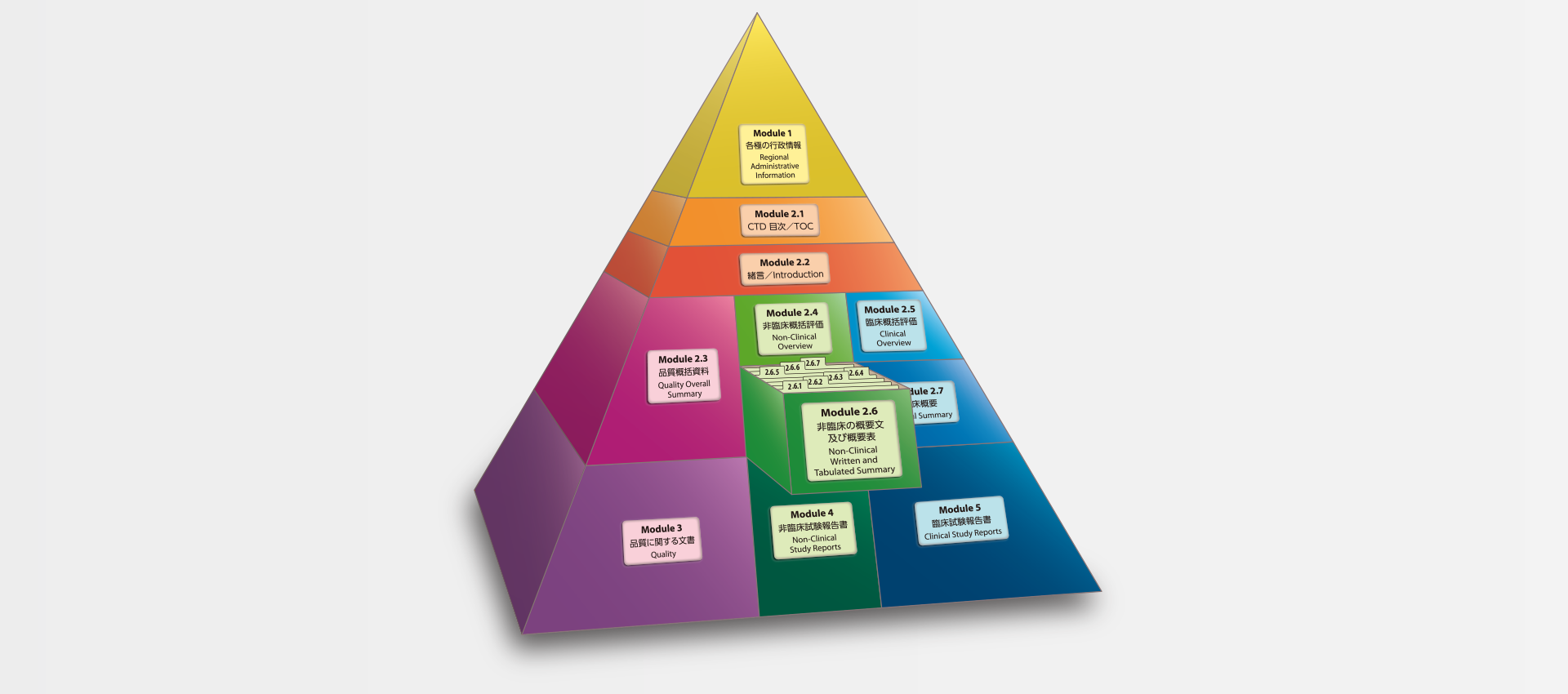
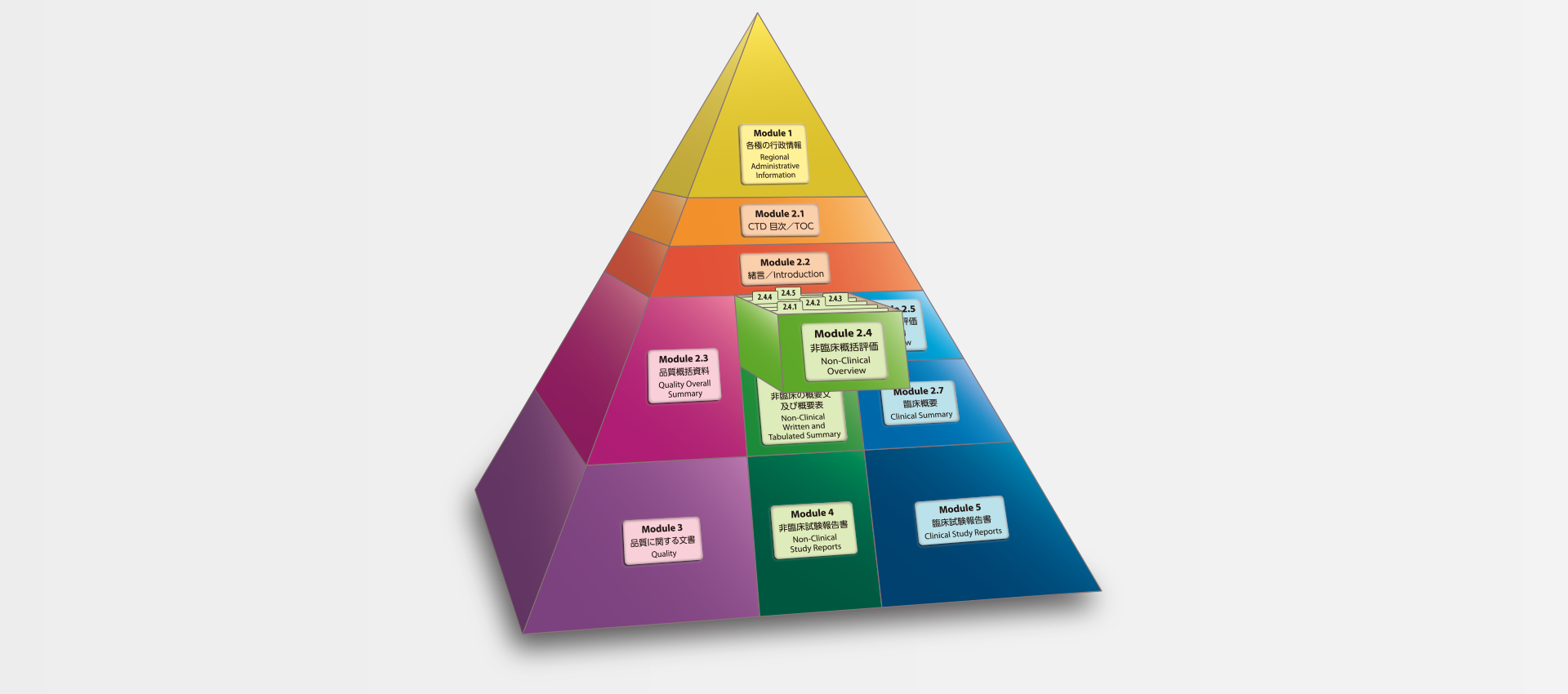
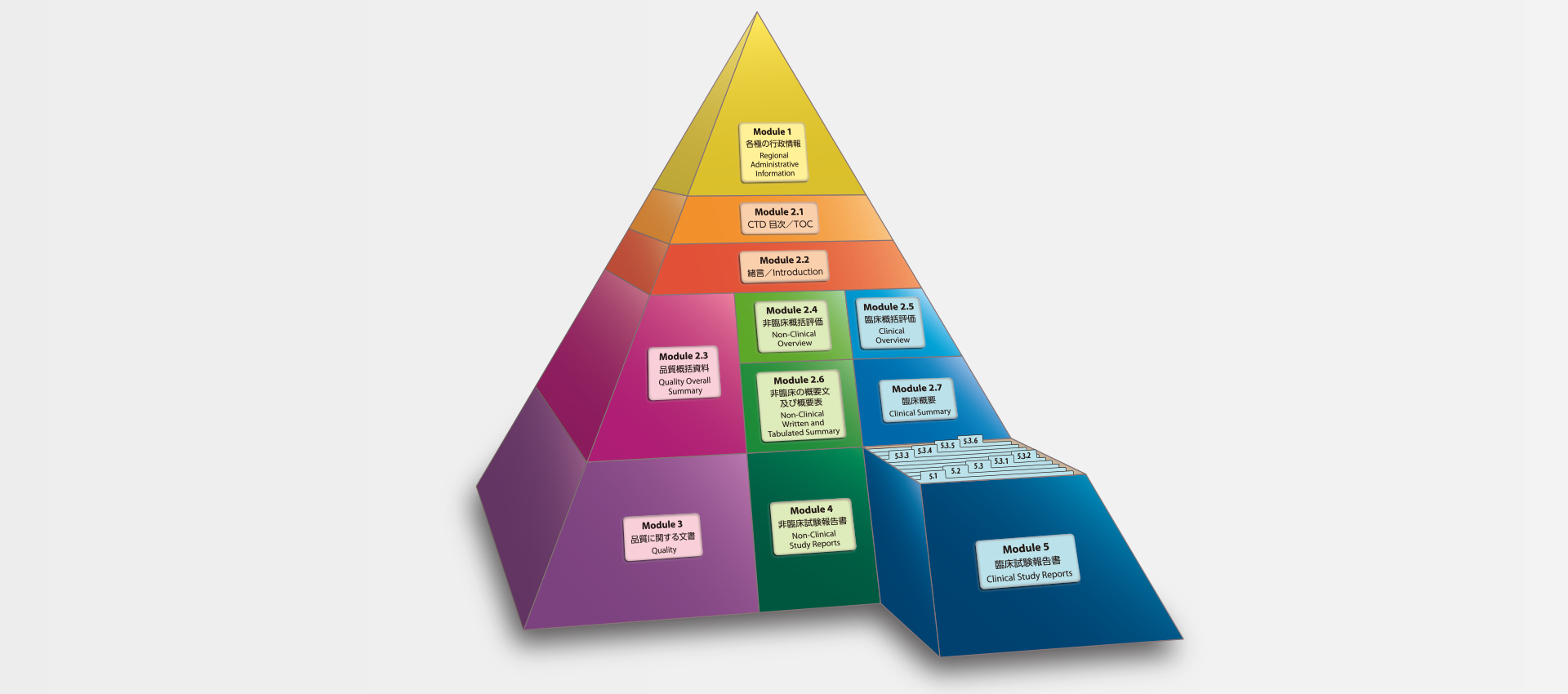
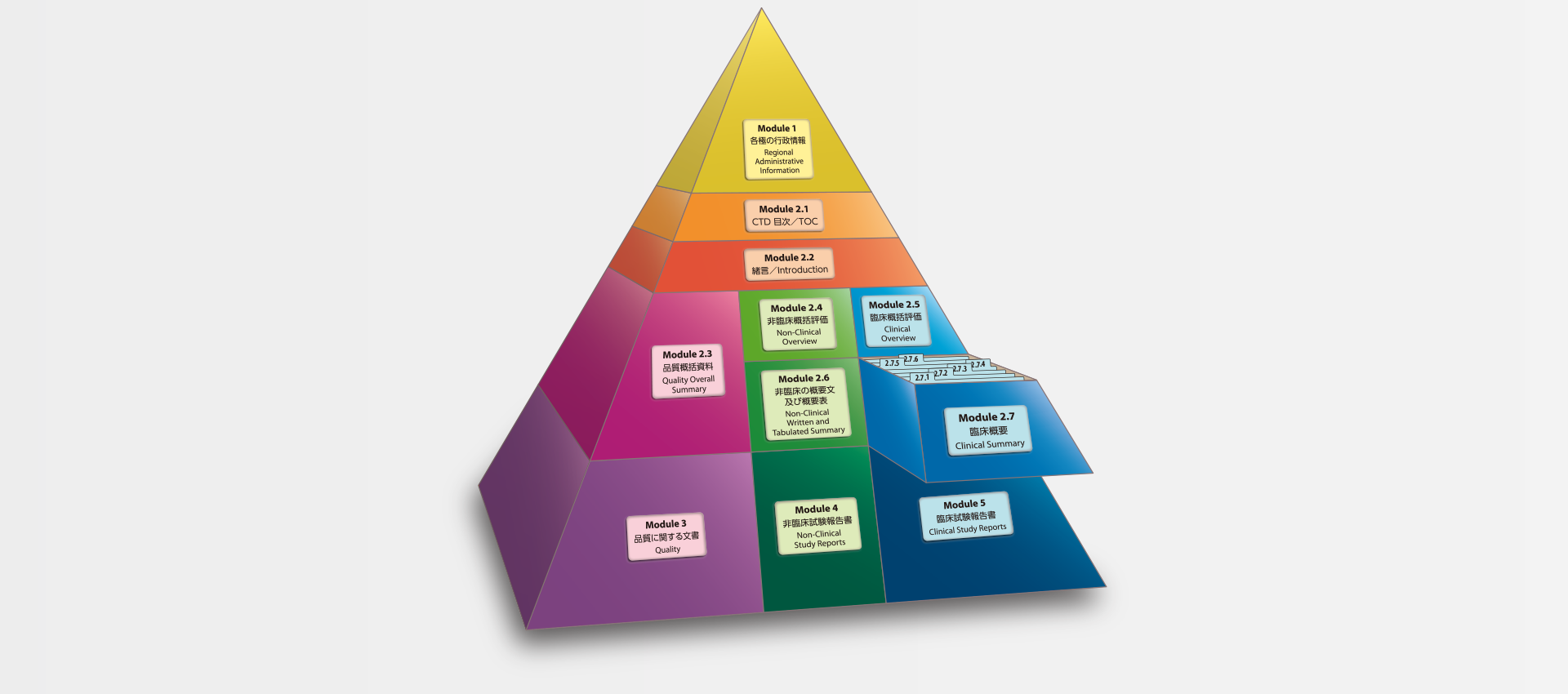
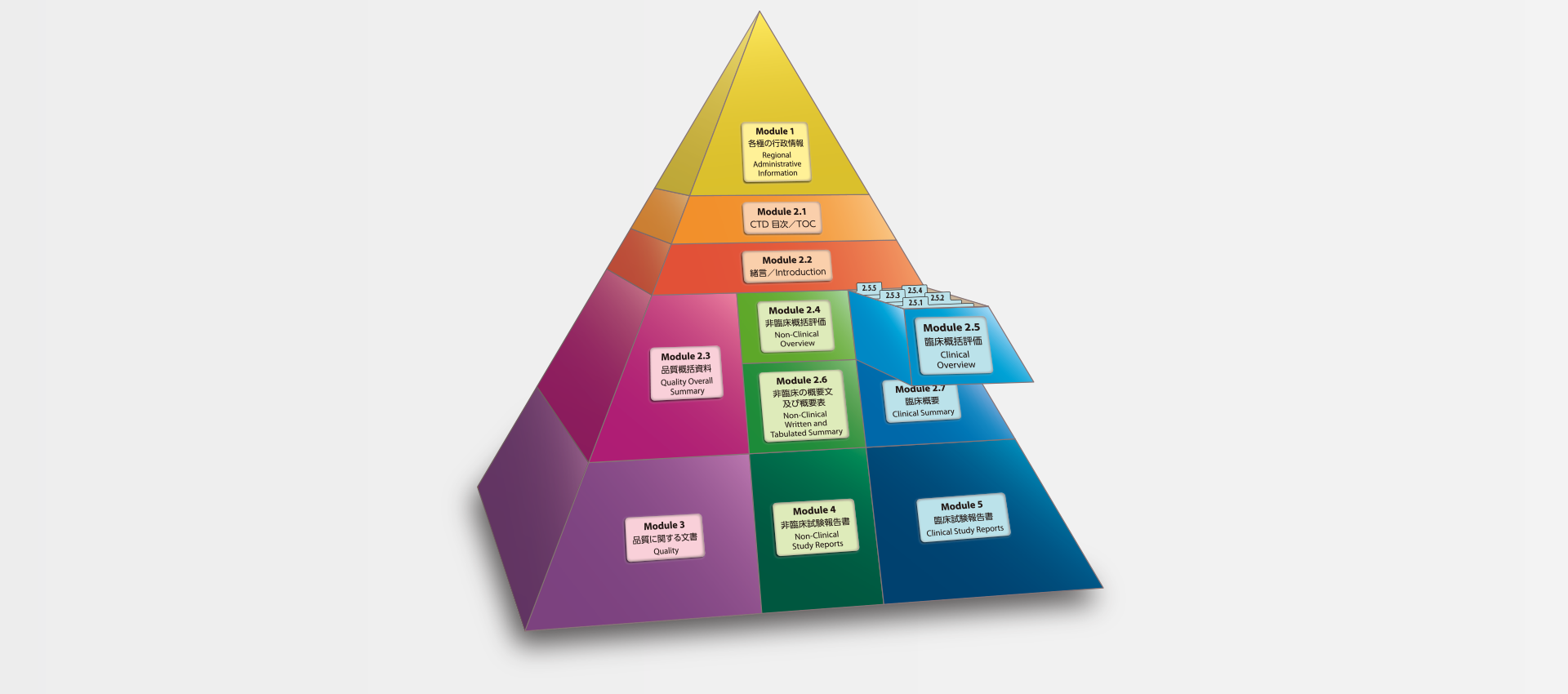
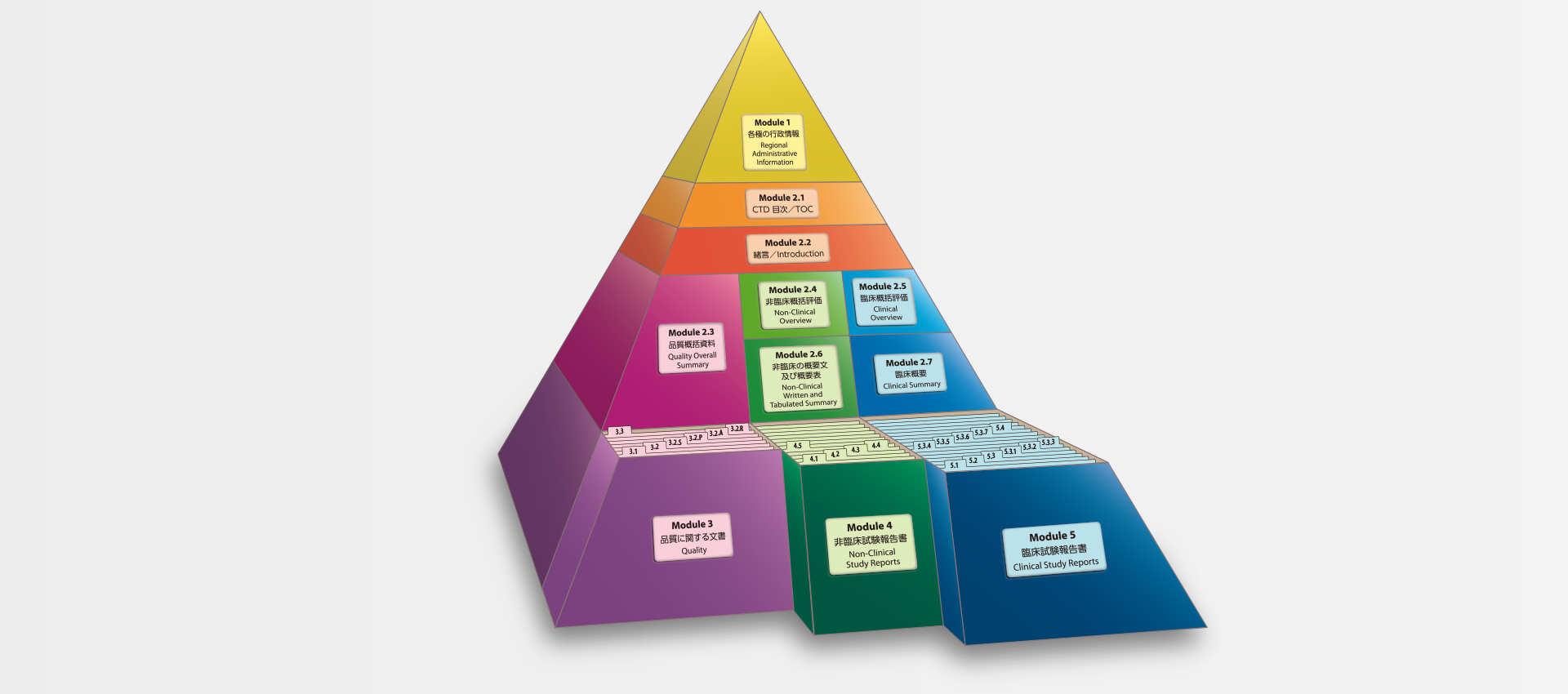
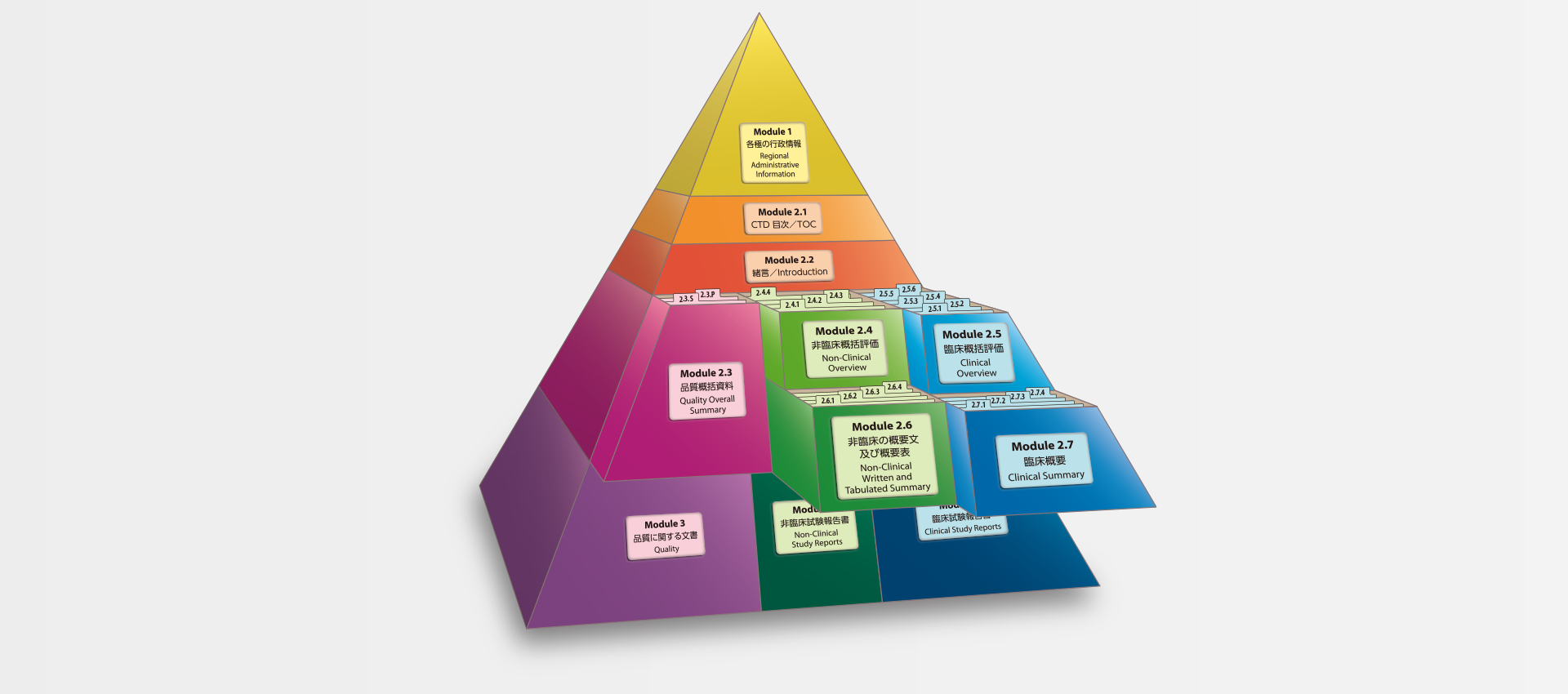
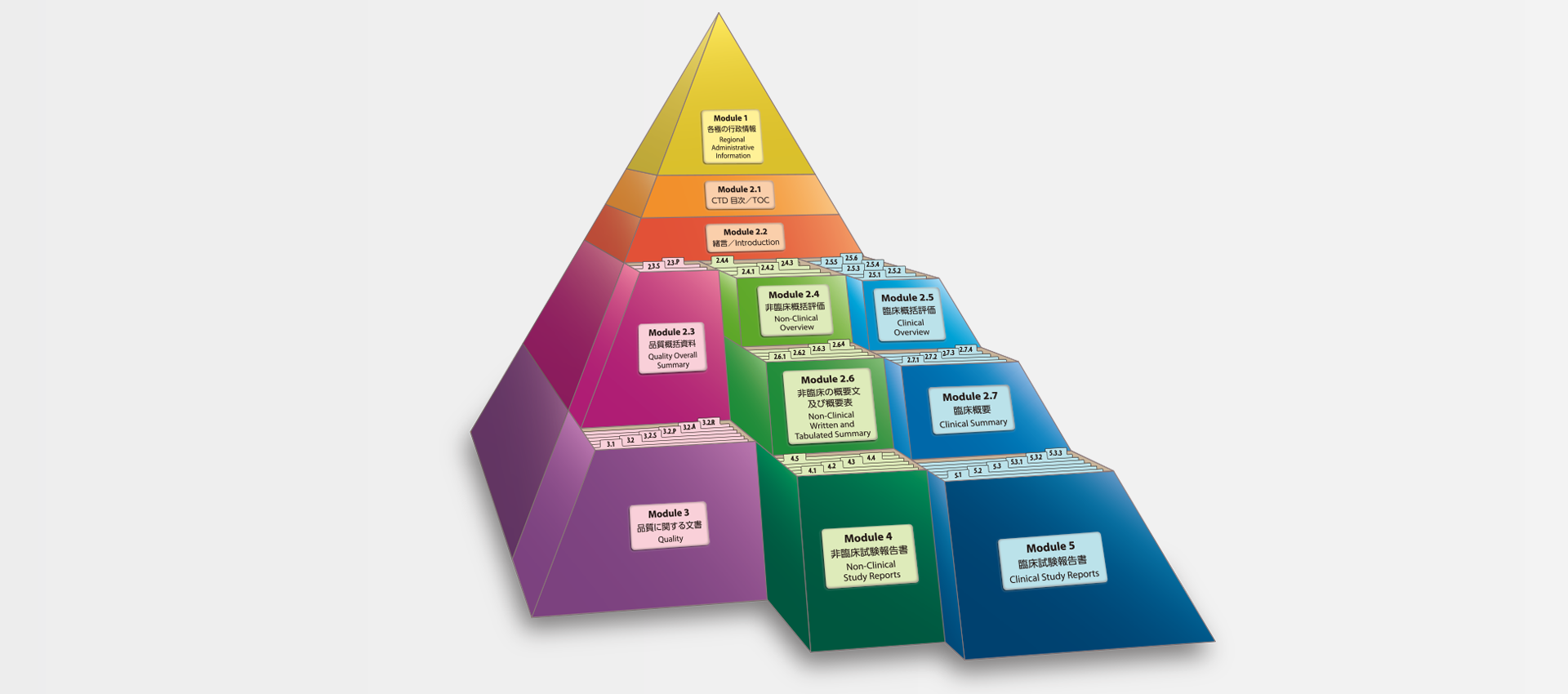
当社では、CTDの5つのモジュール全てにわたる翻訳及びメディカルライティングを行っています。特にモジュール2.5と2.7(臨床)及びモジュール2.4と2.6(非臨床)で実績を積んでおります。
4
治験総括報告書
(CSR)
当社では、CSRの翻訳はもとより、お客様からご提供いただいたプロトコール、SAP、IB、解析結果、CRF、SAE 報告書等を基に、日本語または英語でのCSRを作成しています。国内臨床試験と同時並行で英語版CSRを作成することも可能です。
5
医療機器・バイオ
技術文書
当社では、医療機器分野での翻訳と医療機器承認申請文書のライティングを提供しています。また、バイオテクノロジーの応用領域の翻訳において、最新の知見に基づく正確な翻訳を提供しています。
6
医学論文
当社では、英語ネイティブの医学専門翻訳者が日本語論文を各ジャーナルの投稿規程に準じて英語に翻訳しています。また、当社スタッフが国際的なジャーナルへの論文投稿を全面的にサポートしています。
Business Contents
MedicaLingual utilizes a global network of professionals with a high level of medical and pharmaceutical knowledge and an experience in the field of drug development to provide translation and medical writing service for documents across the entire drug development process, from non-clinical study to new drug application and post-marketing surveillance.
ZYLiQ is a web-based application developed by Symbiance Inc to automate the writing of Clinical Study Reports and Scientific documents with the help of Artificial Intelligence (AI). As a distributor in Japan for ZYLiQ, MedicaLingual provides ZYLiQ presentation, order receipt, delivery, installation support, and user support services in Japanese language.
MedicaLingual has a wealth of experience in the translation and medical writing tasks required for the preparation of all 5 CTD modules, particularly of Modules 2.5 and 2.7 (Clinical) and 2.4 and 2.6 (Non-clinical).
In addition to translating CSRs, MedicaLingual can prepare Japanese-language or English-language CSRs based on protocols, SAPs, IBs, analysis results, CRFs, SAE reports, etc. provided by our clients. We can also prepare English-language CSRs while the Japanese clinical studies are ongoing.
MedicaLingual provides translation in medical device field as well as writing of medical device approval application documents. MedicaLingual also delivers the accurate and up-to-date translations in the application fields of biotechnology.
MedicaLingual provides English translations by Native English-Speaking Professionals in the field of medicine that are fully compliant with the submission instructions of specific journals. MedicaLingual also provides full support for manuscript submission to international journals.
- 2024.04.10
- PMDA’s International Vision in New Mid-t……
- 2024.03.22
- It's A Small World: A Multinational Netw……
- 2024.01.13
- Regulatory Innovation in the Cloud: Acce……
- 2022.04.28
- 本社移転のお知らせ
- 2021.12.28
- 求人情報 / Job Lead